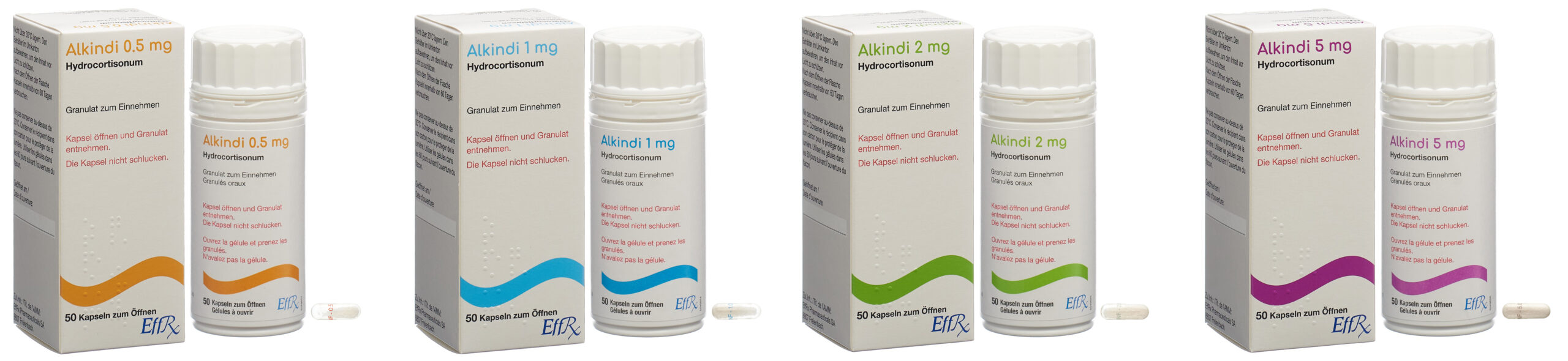
Alkindi® is the first preparation of hydrocortisone specifically designed for and studied in children suffering from pediatric adrenal insufficiency (AI).
Alkindi® is a patented, oral, immediate-release formulation of hydrocortisone-containing granules in capsules for opening. that allows accurate age-appropriate dosing, especially in infants and children. This therapeutic approach has the potential to help young patients under the age of eighteen suffering from pediatric AI and congenital adrenal hyperplasia (CAH).

EffRx obtained the Swiss Marketing Authorization for Alkindi® as a replacement therapy for adrenal insufficiency in infants, children, and adolescents (from birth to less than eighteen years) in 2021. Alkindi® is now also available to patients in Switzerland, where around 200 children suffer from pediatric AI.
For more information, please refer to the medicinal product information published at www.swissmedicinfo.ch