Our pipeline showcases a strategic mix of proprietary products and in-licensed rare disease therapies, highlighting our commitment to improving patient related outcomes through scientific innovation and strategic partnerships.
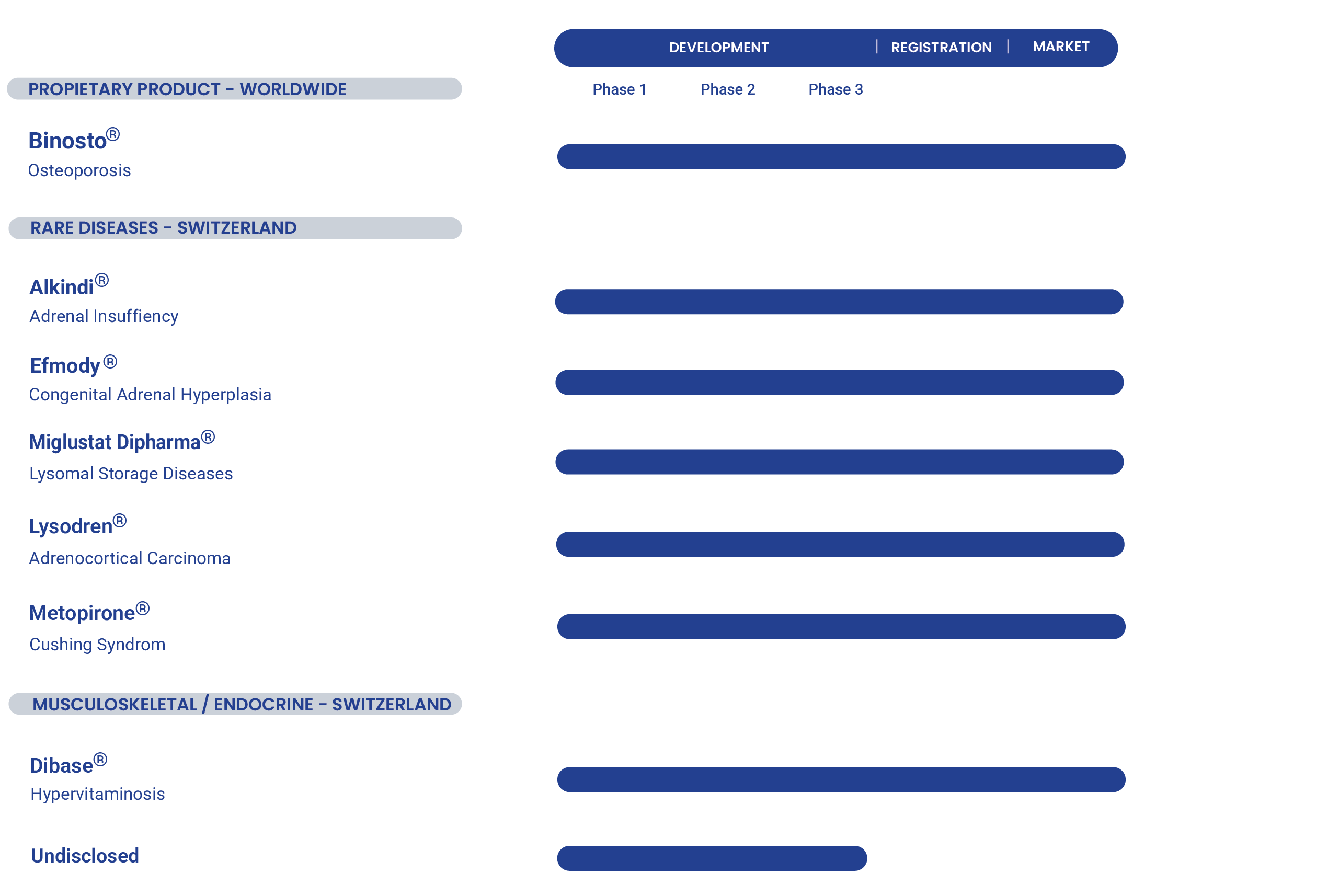
Binosto® (buffered soluble alendronate 70mg) is indicated for the treatment of osteoporosis. Binosto® is an effervescent tablet to be dissolved in half a glass of water and to be taken once a week.
EffRx developed Binosto® from start to finish, including benefit assessment, formulation, clinical development, FDA and European approval, licensing the product for commercialization to national & regional partners around the world.
The approximately 30 marketing authorizations obtained worldwide and the successful launch in as many territories provide an important proof-of-concept of EffRx’s go-to-market capabilities.
Alkindi® is an immediate-release hydrocortisone preparation specifically designed to meet the needs of pediatric patients with adrenal insufficiency.
Efmody® is a modified-release preparation of hydrocortisone specifically designed for the treatment of patients with CAH (Congenital Adrenal Hyperplasia), a rare condition caused by a genetic deficiency of adrenal enzymes.
Miglustat Dipharma is an inhibitor of glucosylceramide synthase, the enzyme responsible for the first step in the synthesis of most glycolipids. Its therapeutic areas are Lysosomal Storage Diseases.